|
 |
| J Med Life Sci > Volume 19(1); 2022 > Article |
|
Abstract
We investigated the safety and efficacy of pemetrexed monotherapy in patients with lung adenocarcinoma and various renal conditions, including chronic kidney disease. We also analyzed whether baseline renal function affected progression-free survival (PFS). We retrospectively analyzed 71 patients who received maintenance-and second-line pemetrexed monotherapy. Patients were divided into two groups according to estimated glomerular filtration rate (eGFR): fair eGFR (>60 mL/min/1.73 m2) and lower eGFR (59 mL/min/1.73 m2 or less). The safety and efficacy were evaluated for each group. Median ages were 73.2 years in the lower eGFR group (n=28) and 64.5 years in the fair eGFR group (n=43). Patients with a lower eGFR achieved a median PFS of 4.7 months, while the median PFS for patients with a fair eGFR was 2.7 months. The difference between the two groups was not statistically significant (p=0.075). Both groups showed treatment-related low-grade hematological and non-hematological adverse events. Pemetrexed monotherapy is safe and effective in patients with lung adenocarcinoma with a lower eGFR.
Lung cancer is a major cause of cancer-related deaths worldwide; mortality due to lung cancer has increased in both sexes in South Korea since 1983 [1]. Lung cancer is generally divided into non-small cell lung cancer (NSCLC), consisting of 80-85% of cases, and small cell lung cancer. Approximately 40% of patients have advanced-stage NSCLC at the time of diagnosis, and thus receive palliative treatment. The current palliative chemotherapeutic strategy for advanced NSCLC is based on histology results and the presence gene mutations such as EGFR, ALK, ROS, and BRAF.
Targeted therapy is the first-line treatment option for patients with actionable mutations. Various targeted therapies and immunotherapies have been developed for the treatment of advanced NSCLC, showing good therapeutic outcomes; nevertheless, the cytotoxicity of chemotherapy remains a major part of cancer treatment [2]. Patients without driver mutations usually receive first-line platinum-based doublet chemotherapy.
Approximately 50% of patients with advanced NSCLC are >70 years of age. Compared with younger patients, these individuals usually have impaired renal function, various comorbidities, a higher risk of chemotherapy-related toxicity, and a shorter life expectancy. These characteristics entail cautious anti-cancer agent selection [3].
In the MILES-3 and MILES-4 studies, patients over 70 years of age with advanced NSCLC with an Eastern Cooperative Oncology Group performance status of 0-1 were randomly assigned to either gemcitabine or pemetrexed groups, with or without cisplatin. The results of this study showed no significant differences in overall survival and hematologic and neurologic toxicities, regardless of cisplatin addition [4].
Pemetrexed is a folate anti-metabolic agent that is excreted from the kidneys at 70-90% and has a half-life of 3.5 hours under normal renal function, with hematologic and non-hematologic toxicity [5]. Variable efficacy on lung cancer was observed, depending on the histological subtype. Pemetrexed has proven to be an effective alternative monotherapy for advanced NSCLC and has been approved by the Food and Drug Association for use during several steps in NSCLC treatment (first-line, maintenance therapy, and second- or later-line therapy) [6]. Superior efficacy was observed in non-squamous cell carcinoma histology compared with squamous histology [7]. Due to the collective drug profile of pemetrexed, it is currently recognized as a chemotherapeutic agent that can be safely used in elderly patients. However, only a few studies have been conducted on pemetrexed use in elderly patients with impaired renal function.
Therefore, our objective was to evaluate the safety and efficacy of pemetrexed across the spectrum of renal function in patients treated with maintenance or second-line pemetrexed monotherapy. In addition, we analyzed whether baseline renal function had an effect on progression-free survival (PFS).
We retrospectively reviewed the medical records of patients with advanced lung adenocarcinoma who received pemetrexed monotherapy at the Department of Hematology and Oncology of Jeju National University Hospital between September 2009 and December 2017. Among the 124 patients with advanced NSCLC, 71 received pemetrexed monotherapy.
The inclusion criteria were as follows: patients aged Ōēź18 years, patients with histologically confirmed non-squamous histology (adenocarcinoma), patients with stage IIIb-IV NSCLC, patients who received pemetrexed monotherapy as maintenance, second-line, and later-line therapy treatment.
Pemetrexed monotherapy is only permitted as a maintenance, second-line, or subsequent line therapy drug under insurance coverage in South Korea. The patients were previously treated with platinum-based doublet or EGFR tyrosine kinase inhibitors chemotherapies. Before the initiation of pemetrexed, simple chest radiography, chest computed tomography (CT), and positron emission tomography-CT (PET-CT) were performed to determine the stage according to the TNM staging system, and the estimated glomerular filtration rate (eGFR, using the modification of diet in renal disease [MDRD] equation) was measured [8]. The study was conducted with the approval of the Institutional Review Board (IRB) of Jeju National University Hospital (IRB No. 2020-12-013), in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patients received pemetrexed monotherapy at 500 mg/m2 in a 10-min intravenous infusion on day 1 of the cycle every 3 weeks. Patients took oral folic acid daily, beginning 1-2 weeks before pemetrexed injection initiation, and continued folic acid daily until 3 weeks after the final pemetrexed dose. Vitamin B12 1,000 mg was injected intramuscularly 1 week before pemetrexed initiation and every 9 weeks until 3 weeks after the final pemetrexed treatment. Cycles were repeated until disease progression, unacceptable toxicity, or therapy discontinuation upon patient or investigator request. In this study, patients were divided using the MDRD equation into the fair eGFR (>60 mL/min/1.73 m2) group and the lower eGFR (59 mL/min/m2 or less) group.
The disease control rate (DCR) was defined as the proportion of patients who experienced a complete response, partial response, or stable disease after treatment. PFS was defined as the time from pemetrexed monotherapy initiation to cancer progression, death from any cause, or last contact with the Clinicians.
To evaluate the effect of pemetrexed on baseline renal function, eGFR was measured before and 3 months after chemotherapy. Clinical evaluation of response was performed using CT, PET-CT, and investigators assessment. Follow-up visits were scheduled every 3 months until disease progression or death. The response to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The degree of toxicity during chemotherapy was evaluated according to the NCI CTC 5.0 criteria.
Based on the K/DOQI guidelines, the stages of renal function were classified into chronic kidney disease (CKD) Stages 1, 2, 3a, 3b, 4, and 5 according to the eGFR: Stage 1 is kidney damage with normal or increased GFR, GFR Ōēź90 mL/min/1.73 m2; Stage 2 is kidney damage with mildly decreased GFR, GFR: 60-89 mL/min/1.73 m2; Stage 3a is mild to moderately decreased GFR, GFR: 45-59 mL/ min/1.73 m2; Stage 3b is moderate to severely decreased GFR, GFR: 30-44 mL/min/1.73 m2; Stage 4 is severely decreased GFR, GFR: 15-29 mL/min/1.73 m2; and stage 5 is kidney failure, GFR <15 mL/min/1.73 m2 (or dialysis) [9].
Statistical analyses were performed using SPSS V13.0 (SPSS Inc., Chicago, IL, USA).
The linear association method was used to determine the response rate (RR) and DCR. The median PFS was analyzed using the chi-squared test and Kaplan-Meier method. The P-value was based on the log-rank test, and statistical significance was set at P<0.05.
A total of 71 patients with lung adenocarcinoma were enrolled in the study from September 2009 to December 2017. The median age was 69.8 years. The median age of the lower eGFR group was 73.2 years and that of the fair eGFR group was 64.5 years. The baseline characteristics of the patients are shown in Table 1.
Before receiving pemetrexed, 60.6% of patients (n=43) had a fair eGFR and 39.4% (n=28) had a lower eGFR. After 3 months of chemotherapy, only two patients in the initial fair eGFR group displayed decreased renal function with moderate to severely decreased eGFR (Table 2). None of the patients required renal replacement therapy until 3 months after receiving pemetrexed monotherapy.
Two deaths occurred during pemetrexed monotherapy. One patient died from complications related to infection, the other from the progression of lung cancer. The association with chemotherapy was not clear in either case.
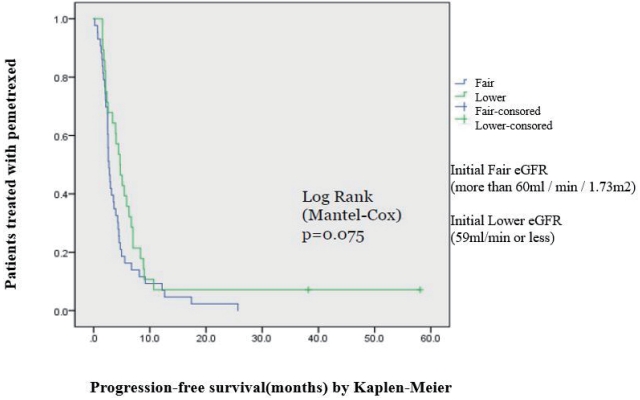
Comparing the response between the two groups, the median PFS was 2.7 months (95% confidence interval [CI], 2.3-3.1) in the fair eGFR group, and 4.7 months (95% CI, 3.3-6.0) in the lower eGFR group. There were no statistically significant differences between the two groups (P=0.075) (Fig. 1).
The RRs for the fair and low eGFR groups were 7% and 17.9%, respectively. The DCR was 53.5% and 39.3% in the fair and low eGFR groups, respectively. We hypothesize that the difference is due to the large number of participants in the fair eGFR group; however, there was no statistically significant difference between the groups in terms of response and disease control rates (P=0.156 and P=0.242, respectively) (Table 3).
A comparison of treatment-related adverse events is presented in Table 4. When comparing hematologic toxicity, neutropenia occurred only in the lower eGFR group. Grade 2 anemia occurred in eight (18%) and 10 patients (35%) in the fair and low eGFR groups, respectively. Thrombocytopenia occurred in one (2.3%) and five patients (17.8%) in the lower and fair eGFR groups, respectively. Fatigue was the most common adverse event observed in both groups. Nineteen patients (44.1%) in the fair eGFR group and 14 (50%) in the lower eGFR group experienced fatigue. Other non-hematologic adverse events observed included nausea, vomiting, diarrhea, rash, and neuropathy. However, no Grade 3 or 4 treatment-related adverse events were observed in either group (Table 4). No treatment was discontinued due to toxicity.
Most patients with advanced lung adenocarcinoma develop progressive disease or relapse after first-line therapy and receive second-line treatment [10]. However, second-line therapy in patients with advanced lung adenocarcinoma and CKD is often difficult to select due to treatment-related toxicity. Therefore, an effective anticancer agent with low toxicity is necessary. Pemetrexed is often chosen for its relatively lower toxicity, even in elderly patients.
In this study, the median age of the lower eGFR group was 73.2 years and that of the fair eGFR group was 64.5 years. In the lower eGFR group, the RR and DCR were 17.9% and 39.3%, respectively, and 7% and 53.5% in the fair eGFR group, respectively. After 3 months of pemetrexed therapy, only 5% of the patients in the initial fair eGFR group experienced renal impairment. Compared with treatment-related toxicity, both groups showed manageable low-grade hematologic toxicity and non-hematologic toxicity.
This study had some limitations. First, eGFR estimates were calculated by MDRD equation which was not gold standard for measuring GFR. The gold standard measurement of GFR is using urinary or plasma clearance of an exogenous filtration marker, such as inulin. However, this gold standard measurement is unsuitable for clinical settings [11,12]. Second, we retrospectively reviewed the medical records of a single center, and the sample size was limited to 71 patients. However, few previous studies have focused on the safety of pemetrexed in patients with CKD as we have. Therefore, we believe that this study can serve as a meaningful starting point for preliminary data needed to design follow-up studies.
In conclusion, pemetrexed monotherapy may be used as a relatively safe maintenance, second-line, or subsequent-line therapy option in patients with lung adenocarcinoma, even in those with chronic kidney disease, without dose reduction or loss of therapeutic effect. This finding also appears to be valid in elderly patients.
Approximately 40% of patients with advanced-stage NSCLC are over 70 years of age and usually have comorbidities, including impaired renal function.
Pemetrexed is used for the treatment of NSCLC and is primarily excreted by the kidneys.
Pemetrexed monotherapy is safe and effective in elderly patients with lung adenocarcinoma with a lower eGFR.
Figure┬Ā1.
Progression-free survival of patients treated with pemetrexed with an initial fair eGFR vs. those with a lower eGFR. eGFR: estimated glomerular filtration rate.
Table┬Ā1.
Baseline characteristics of patients
Table┬Ā2.
Initial GFR and the GFR in patients after 3 months (GFR3) of pemetrexed therapy
| Initial GFR |
GFR after 3 months |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | Total | |
| 1 | 5 | 4 | 0 | 0 | 0 | 0 | 9 |
| 2 | 9 | 20 | 5 | 0 | 0 | 0 | 34 |
| 3a | 0 | 7 | 15 | 2 | 0 | 0 | 24 |
| 3b | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| 4 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 14 | 32 | 21 | 3 | 1 | 0 | 71 |
Table┬Ā3.
Clinical response after 3 months of pemetrexed in the context of eGFR
| RR* | DCRŌĆĀ | CR | PR | SD | PD | |
|---|---|---|---|---|---|---|
| Fair eGFR (n=43) | 3 (7.0) | 23 (53.5) | 0 (0.0) | 3 (7.0) | 20 (46.5) | 20 (46.5) |
| Lower eGFR (n=28) | 5 (17.9) | 11 (39.3) | 0 (0.0) | 5 (17.9) | 6 (21.4) | 17 (60.7) |
Table┬Ā4.
Treatment-related adverse events of pemetrexed chemotherapy
REFERENCES
2. Cortinovis D, Abbate M, Bidoli P, Capici S, Canova S. Targeted therapies and immunotherapy in non-small-cell lung cancer. Ecancermedicalscience 2016;10:648.



3. Pirker R. Systemic therapy of elderly patients with advanced nonsmall cell lung cancer-individualized treatment is key. Ann Transl Med 2019;7(Suppl 1):S48.



4. Gridelli C, Morabito A, Cavanna L, Luciani A, Maione P, Bonanno L, et al. Cisplatin-based first-line treatment of elderly patients with advanced non-small-cell lung cancer: joint analysis of MILES-3 and MILES-4 phase III trials. J Clin Oncol 2018;36:2585-92.


5. de Rouw N, Boosman RJ, van de Bruinhorst H, Biesma B, van den Heuvel MM, Burger DM, et al. Cumulative pemetrexed dose increases the risk of nephrotoxicity. Lung Cancer 2020;146:30-5.


6. Hata A, Katakami N, Hattori Y, Tanaka K, Fujita S, Kotani Y, et al. Pemetrexed monotherapy for chemo-na├»ve elderly (aged Ōēź80) patients with non-squamous non-small cell lung cancer: results from combined analysis of two single arm phase II studies (HANSHIN002 and 003). Cancer Chemother Pharmacol 2017;79:689-95.


7. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.


8. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70.


9. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1-266.

10. Bluthgen MV, Besse B. Second-line combination therapies in nonsmall cell lung cancer without known driver mutations. Eur Respir Rev 2015;24:582-93.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print