|
 |
| J Med Life Sci > Volume 20(2); 2023 > Article |
|
Abstract
Multiple studies have reported on unilateral axillary adenopathy following coronavirus disease 2019 (COVID-19) vaccination, which is currently recognized as a common finding. Here, we present a series of eight adult patients with reactive axillary lymphadenopathy following COVID-19 vaccination, in whom the follow-up ultrasonography (US) showed resolution of a previously noted unilateral axillary adenopathy. From March 2021 to March 2022, 2,599 consecutive women underwent breast US in Jeju National University Hospital. We identified 10 patients with unilateral axillary lymphadenopathy following COVID-19 vaccination detected on the breast US. The 10 patients were recommended for follow-up US. Two patients were lost to follow-up, whereas the remaining eight patients underwent follow-up US, in whom resolution of the unilateral axillary lymphadenopathy was noted. Radiologists should be aware of evolving guidelines for evaluating and managing axillary lymphadenopathy to avoid false positive biopsies. Recent studies on lymphadenopathy following COVID-19 vaccination show that a prolonged duration until resolution is often observed. Therefore, a follow-up US examination at least 12 weeks after vaccination may be reasonable. Furthermore, management guidelines should include a risk-stratified approach considering both vaccination timing and the patientŌĆÖs overall risk of metastatic disease.
Unilateral axillary lymphadenopathy is a well-known side effect of coronavirus disease 2019 (COVID-19) vaccination. We have frequently encountered patients with unilateral axillary lymphadenopathy on diagnostic and screening breast ultrasonography (US) for various purposes. Reactions to the Pfizer-BioNTech and Moderna vaccinations are common, with the most common unsolicited adverse event reported being unilateral axillary swelling and tenderness. These occur following first and second Moderna injections in 10.2% and 14.2% of recipients, respectively [1]. During clinical trials for the Pfizer-BioNTech COVID-19 vaccine, the self-reported rate of unilateral axillary and supraclavicular lymphadenopathy among vaccine recipients was 0.3%, with symptoms developing 2-4 days after vaccination and lasting approximately 10 days. 2 In clinical trials of the Moderna COVID-19 vaccination, the incidence of clinically apparent lymphadenopathy was 1.1%, with symptoms developing within 2-4 days after vaccination and a median duration of 1-2 days [3]. Since these trials only reported the presence of clinically symptomatic axillary swelling or tenderness and palpable lymph nodes, the actual frequency of lymphadenopathy following COVID-19 vaccination might be underestimated. Since March 2021, multiple case series on COVID-19 vaccine-induced adenopathy have been published [4-9]. The incidence of imaging-detected adenopathy is much greater than that of clinically detected adenopathy, with retrospective studies reporting ranges of 2.4-35% for axillary lymphadenopathy in women undergoing screening mammogram and/or US [10,11].
Initially, the Society of Breast Imaging (SBI) recommended scheduling a screening mammogram before a first vaccine dose or 4-6 weeks after the second dose of COVID-19 vaccine [12]. The SBI proposed further guidelines on managing presumed COVID-19 vaccination-induced axillary lymphadenopathy in 2021, recommending that short-term imaging follow-up at 4-12 weeks should be performed. Recently, as clinical experience has provided information on the duration and incidence of axillary lymphadenopathy following COVID-19 vaccination, the recommendations have evolved. Wolfson et al. [10] reported that the time to resolution of reactive lymphadenopathy varies, with persistent lymphadenopathy being detected up to 43 weeks following vaccination. Additionally, they suggested that a delay in screening mammogram due to the extended time to resolution should be avoided [10]. In March 2022, the SBI revised its guidelines to recommend a follow-up interval of 12 weeks or more because of the recognition that COVID-19 vaccination-related lymphadenopathy can persist as late as 71 days or 43 weeks after vaccination [10,11,13]. They suggested considering a potential breast imaging and reporting and data system (BI-RADS) category 2 assessment in asymptomatic patients without risk of metastatic lymphadenopathy and no suspicious breast imaging, as recommended by other studies [14-16].
Axillary lymphadenopathy is not unique to the COVID-19 vaccine and can occur shortly after receiving any vaccination. Rare cases of unilateral axillary adenopathy related to recent vaccination were reported following administration of the smallpox, Bacille Calmette-Gu├®rin, influenza, and human papillomavirus vaccines [17]. Axillary adenopathy is more common after messenger RNA (mRNA) vaccines than after protein-based vaccines because mRNA vaccines evoke very strong and rapid B-cell proliferation in the germinal center of lymph nodes [18,19]. Therefore, vaccine-induced axillary lymphadenopathy on imaging is likely to occur more frequently with COVID-19 vaccinations than with other vaccinations [1].
In this report, we presented a series of eight adult patients with reactive axillary lymphadenopathy following COVID-19 vaccination, in whom follow-up US showed resolution of a previously noted unilateral axillary adenopathy. We also provided a review of the relevant literature.
This retrospective study was approved by the Institutional Review Board of Jeju National University Hospital, and the requirement for informed consent was waived. From March 2021 to March 2022, 2,599 consecutive women underwent breast US in our institution. Here, we identified 10 patients with unilateral axillary lymphadenopathy following COVID-19 vaccination detected on breast US. We then included eight patients who presented for follow-up breast US in our case series. The clinical information on age, gender, and axillary symptom and sign was obtained from the electronic medical record or directly from the patient at the time of examination. Additionally, questionnaires were used to gather information including vaccination timing, type, and site of COVID-19 vaccination before performing US.
Clinical informaion and radiologic features of the eight patients are shown on Table 1.
A 41-year-old woman presented for routine mammography screening and ultrasonographic follow-up of probably benign bilateral breast nodules. The woman had no personal or family history of breast cancer. Mammography demonstrated a prominent lymph node in the left axilla measuring 15 mm in the longest diameter (Fig. 1A). The remaining area of the bilateral breast and axilla was unremarkable. Breast and axillary US demonstrated multiple enlarged axillary lymph nodes in the left axilla. The largest node reached up to 23 mm in the longest diameter with a cortical thickness of 9 mm (Fig. 1B), along with stable and newly identified probably benign nodules in the bilateral breast and no abnormal lymph nodes in the right axilla. Upon interview, the patient revealed that she had received the first dose of Pfizer-BioNTech COVID-19 vaccine in her left upper extremity 13 days prior. The patient also suffered from pain affecting the left upper arm and left axillary area. Based on this information, axillary lymphadenopathy was attributed to the recent vaccination. The patient was advised to undergo short-term follow-up breast US for the probably benign bilateral breast nodules to ensure resolution of the enlarged left axillary lymph node. Follow-up US obtained after 6 months showed a cortical thickness of 3 mm, consistent with resolution of the lymphadenopathy (Fig. 1C).
A 48-year-old woman with no personal or family history of breast cancer presented for routine screening mammography and sonographic follow-up of probably benign bilateral breast nodules. Breast US demonstrated multiple enlarged axillary lymph nodes in the right axilla, the largest reaching up to 19├Ś6├Ś15 mm with diffuse cortical thickening of 5 mm and preservation of fatty hilum (Fig. 2A). The patient had received the second dose of Pfizer-BioNTech COVID-19 vaccine in her right upper extremity 22 days prior. Therefore, these findings were attributed to the recent vaccination. Additionally, a 1.6 cm nodule categorized as BI-RADS 4a was detected. The US-guided core needle biopsy was performed, leading to a diagnosis of intraductal papilloma. The remaining area of bilateral breasts and left axilla was unremarkable. The patient was advised to undergo short-term follow-up breast US for the probably benign bilateral breast nodules. Follow-up US obtained after 6 months showed a cortical thickness of 3 mm, consistent with resolution of the lymphadenopathy (Fig. 2B).
A 44-year-old woman with no personal or family history of breast cancer was evaluated for breast pain. Breast and axillary US revealed multiple cysts of various sizes, complicated cysts in bilateral breasts, and multiple enlarged lymph nodes in the left axilla. The largest lymph node reached up to 12├Ś7├Ś10 mm with uniform cortical thickening of 6 mm (Fig. 3A). The patient had received the second dose of Pfizer-BioNTech COVID-19 vaccine in her left upper extremity 21 days prior. Therefore, these findings were attributed to the recent vaccination. The patient was advised to undergo short-term follow-up breast US for the probably benign bilateral breast nodules. Follow-up US obtained after 6 months showed cortical thickness of 4 mm, consistent with resolution of the lymphadenopathy (Fig. 3B).
A 41-year-old woman with a history of microinvasive carcinoma in her right breast presented for routine follow-up mammography and US. The patient underwent right mastectomy 3 years prior. Mammography revealed a newly identified low-suspicion microcalcification in the left upper outer breast that was categorized as BI-RADS 4a without a US correlate. Additionally, US of the left axilla showed lymph nodes measuring 10├Ś9├Ś10 mm in size with uniform cortical thickening of 6 mm (Fig. 4A). Although the low-suspicion microcalcification in the left upper outer breast was found on mammogram, the axillary lymph nodes showed reniform morphology and preserved fatty hila. The patient had received the second dose of Pfizer-BioNTech COVID-19 vaccine in her left upper extremity 4 days prior. Thus, axillary lymphadenopathy was attributed to the recent vaccination. The patient underwent mammogram-guided needle localization with surgical biopsy to obtain histological diagnosis of the mammographic screen-detected microcalcification. The microcalcification was diagnosed as ductal carcinoma in situ (DCIS) on histopathologic examination, which was in concordant with the radiological findings. Wide local excision was performed, and DCIS was diagnosed on the final histopathological examination. Follow-up US obtained after 6 months showed a cortical thickness of 1.5 mm, consistent with resolution of the lymphadenopathy (Fig. 4B).
A 44-year-old woman with no personal or family history of breast cancer was referred from an outside institution for US-guided core needle biopsy of a suspicious breast lesion. Breast US showed a 0.7 cm nodule in the left upper outer breast that was categorized as BI-RADS 4a. US-guided core needle biopsy was performed, leading to a diagnosis of fibroadenoma. Axillary US revealed multiple enlarged axillary lymph nodes in the left axilla at levels 1 and 2; the largest node was 17├Ś9├Ś14 mm in size with cortical thickening of 7 mm. Upon further interview, the patient reported receiving the second dose of Pfizer-BioNTech COVID-19 vaccine in her left upper extremity 26 days prior. Hence, these findings were attributed to the recent vaccination. Follow-up US obtained after 6 months showed a cortical thickness of 1.7 mm in the left axilla at level 1, consistent with resolution of the lymphadenopathy.
A 54-year-old woman with no personal or family history of breast cancer presented for routine screening mammography and sonographic follow-up of probably benign bilateral breast nodules. Mammography showed clustered microcalcifications in the left upper outer breast, which were categorized as BI-RADS 3 without a US correlate. Breast and axillary US revealed multiple enlarged axillary lymph nodes in the left axilla, the largest reaching up to 8├Ś10├Ś12 mm with uniform cortical thickening of 6 mm in left axilla level 1 (Fig. 5A, B). Upon interview, the patient reported receiving the first dose of Moderna vaccine in her left upper extremity 13 days prior. Therefore, this finding was attributed to the recent vaccination. Additionally, a 1 cm nodule categorized as BI-RADS 4a was detected in her right upper outer breast. US-guided core needle biopsy was performed, leading to a diagnosis of fibrocystic change. The remaining area of bilateral breasts and left axilla was unremarkable. Follow-up US obtained after 6 months showed a cortical thickness of 2.5 mm, consistent with resolution of the lymphadenopathy (Fig. 5C, D).
A 50-year-old woman with a history of invasive carcinoma of the right breast presented for routine follow-up mammography and US. She had undergone right partial mastectomy 5 years prior. A routine follow-up US showed two new enlarged left axillary lymph nodes at levels 1 and 2, measuring 16├Ś10├Ś16 mm with uniform cortical thickening of 9 mm and 34├Ś9├Ś15 mm with uniform cortical thickening of 4 mm, respectively (Fig. 6A, B). Further investigation revealed that the patient had received the first dose of Moderna vaccine in her left upper extremity 14 days prior. Therefore, this finding was attributed to the recent vaccination. The remaining area of bilateral breasts and right axilla was unremarkable except for stable, probably benign nodules in the bilateral breasts. Follow-up US obtained after 6 months showed a cortical thickness of 2 mm, consistent with resolution of the lymphadenopathy (Fig. 6C, D).
A 30-year-old woman with no personal or family history of breast cancer was referred from an outside institution for US-guided core needle biopsy of a suspicious breast lesion. Breast US demonstrated a 1 cm nodule in the right upper central breast that was categorized as BI-RADS 4a. US-guided core needle biopsy was performed, leading to a diagnosis of fibroadenomatous change. Axillary US showed multiple enlarged axillary lymph nodes in the right axilla at levels 1 and 2, the largest reaching up to 23├Ś6├Ś8 mm with cortical thickening of 5 mm (Fig. 7A, B). Upon further interview, the patient reported receiving the first dose of OxfordAstraZeneca COVID-19 vaccine in her right upper extremity 2 days prior. Therefore, these findings were attributed to the recent vaccination. Follow-up US obtained after 6 months showed a cortical thickness of 1.8 mm, consistent with resolution of the lymphadenopathy (Fig. 7C, D).
In the setting of widespread COVID-19 vaccination, vaccine-induced axillary adenopathy is frequently encountered by breast radiologists [20]. Vaccination-induced adenopathy can result in lymph node enlargement in the unilateral axillary and supraclavicular regions because of the migration of antigen-presenting cells from the injection site to draining nodes, where they elicit cellular (T-cell) and humoral (B-cell) immune responses [18,19]. On US imaging, postvaccination immune response activation may show hyperplastic axillary adenopathy and cortical thickening of lymph nodes unilateral to the vaccine administration site.
The differential diagnoses of axillary lymphadenopathy without an apparent abnormality in the breast include benign reactive nodules, connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus, systemic inflammatory processes (sarcoidosis), infections (bacterial lymphadenitis and tuberculosis), hematologic malignancies, and occult carcinomas [21].
Several recent studies reported on the frequency of vaccine-related lymphadenopathy on breast imaging [10,13,22]. Wolfson et al. [10] found that 44% of patients who received COVID-19 vaccination and underwent breast imaging showed lymphadenopathy on at least one breast imaging modality, with 9% of these found on mammography alone, 61% on US alone, and 30% on both modalities. According to a retrospective review of 951 patients who underwent 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) after vaccination in Israel, 45.6% of the patients had hypermetabolic lymphadenopathy, and only 5.1% of these patients have malignant nodes [23]. Another study on FDG PET-CT scans for oncologic indications found axillary lymphadenopathy following COVID-19 vaccination in up to 15% and 57% of patients who received Pfizer-BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine, respectively [7].
Several studies reported on the expected time course for resolution of lymphadenopathy [10,13,24,25]. In the largest study on axillary lymphadenopathy following COVID-19 vaccination with long-term follow-up of 6 months, the time to resolution of reactive lymphadenopathy was variable, with persistent lymphadenopathy observed up to 43 weeks following vaccination. Lymphadenopathy was found as early as 1 day after the first dose of vaccine and as late as 71 days after the second dose [10]. Therefore, a long resolution time for lymphadenopathy should not be hastily attributed to metastasis, and short-term follow-up imaging may not be necessary. Lane et al. [13] found a mean time to lymphadenopathy resolution of 97 days from the initial US showing lymphadenopathy, and 127 days from the first vaccine dose. Another recent prospective study by Ha et al. [25] found that 26% of axillary lymphadenopathy cases showed complete resolution on follow-up US at a median of 6 weeks after vaccination, and 51% of axillary lymphadenopathy cases persisted on follow-up US at a median of 12 weeks. These results suggest that a follow-up interval of at least 12 weeks for suspected vaccination-induced lymphadenopathy may be reasonable. Furthermore, variability in the degree of lymphadenopathy according to the vaccine type has been reported. A higher incidence and longer duration of lymphadenopathy with a gradual decrease over time on US were noted in recipients of mRNA vaccine [25,26]. Faermann et al. [8] reported an analysis of 163 cases of COVID-19 vaccination-induced lymphadenopathy from a relatively large clinical study and showed that the cortical thickness of lymph node was thinner at 4-5 weeks post vaccination. A systematic review found that the mean size of vaccination-induced axillary adenopathy was 18.2 mm (range, 16-21), and the mean duration from vaccination to adenopathy occurrence was 6.9 days (range, 2-18) [27]. Recently, Ha et al. [25] prospectively evaluated temporal changes in US findings of COVID-19 vaccination-induced lymphadenopathy and found that all patients showed unilateral axillary lymph nodes with diffuse or focal cortical thickening greater than 3 mm, and that a fatty hilum was preserved in most cases. The largest cortical thickness was observed within 2 and 2-4 weeks following mRNA and vector vaccine (AstraZeneca) administration, respectively. Moreover, lymph node cortical thickness values were higher in recipients of mRNA vaccines than that in recipients of vector vaccine (AstraZeneca). Additional suspicious morphological features such as fatty hilum loss, rounded hypoechoic features, increased cortical flow, and an increase in the overall number of lymph nodes were more frequently observed in recipients of mRNA vaccines [25].
A study on FDG uptake of axillary lymph nodes after COVID-19 vaccination described avid axillary lymph nodes in 54% of oncology patients. Here, a mean maximum standardized uptake value of 5.1 and a higher frequency were noted in recipients of the Moderna vaccine than those in recipients of the Pfizer-BioNTech vaccine (72% vs. 43%) [28]. Even in lymph nodes with a normal size, abnormal nodal uptake may persist for a long time on FDG PET-CT after COVID-19 vaccination [16]. Another study reported that mRNA vaccines can induce persistent FDG uptake even at 7-10 weeks after the second dose [29].
Asymptomatic patients with unilateral axillary lymphadenopathy in a setting of recent (<6 weeks) unilateral COVID-19 vaccination without current cancer or abnormal imaging findings may be clinically followed to resolution, and a recommendation for follow-up imaging may not be warranted [1,14,30]. For symptomatic patients with palpable axillary adenopathy in a setting of recent unilateral vaccination, clinical follow-up may be recommended. US can be recommended if clinical concern persists 6 weeks after receiving the last dose of COVID-19 vaccination [1]. A recent study found that all patients diagnosed with axillary metastases had suspicious imaging findings in the unilateral breast or a known cancer history; therefore, concomitant suspicious mammographic findings in the unilateral breast and individual risk factors should be considered when assessing the lymphadenopathy [10].
In COVID-19 pandemic era with ongoing implementation of booster vaccines, radiologists should be aware of evolving guidelines for evaluating and managing axillary lymphadenopathy to avoid false positive biopsies. Recent studies on lymphadenopathy following COVID-19 vaccination have shown that a prolonged duration until resolution is often observed, with lymphadenopathy persisting for 6 weeks. Therefore, a follow-up US examination at least 12 weeks after vaccination may be reasonable. Furthermore, management guidelines should follow a risk-stratified approach considering both vaccination timing and the patientŌĆÖs overall risk of metastatic disease.
Figure┬Ā1.
A 41-year-old woman with unilateral axillary lymphadenopathy after the first dose of Pfizer-BioNTech coronavirus disease 2019 vaccine. (A) Routine mammography obtained within 13 days of vaccination shows a prominent lymph node in the left axilla measuring 15 mm in the longest diameter (arrow). (B) Axial ultrasonography images of the left axilla obtained within 13 days of vaccination show the cortex measuring up to 9 mm (arrow). (C) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 3 mm (arrow).
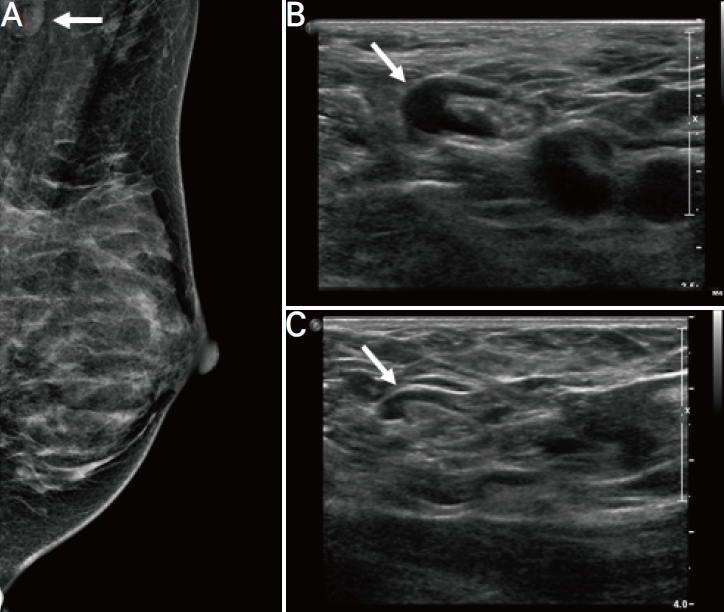
Figure┬Ā2.
A 48-year-old woman with unilateral axillary lymphadenopathy after the first dose of Pfizer-BioNTech coronavirus disease 2019 vaccine. (A) Axial ultrasonography (US) images of the right axilla obtained within 22 days of vaccination show the cortex measuring up to 5 mm (arrow). (B) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 3 mm (arrow).

Figure┬Ā3.
A 44-year-old woman with unilateral axillary lymphadenopathy after the first dose of Pfizer-BioNTech coronavirus disease 2019 vaccine. (A) Axial ultrasonography (US) images of the left axilla obtained within 21 days of vaccination show the cortex measuring up to 6 mm (arrow). (B) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 4 mm (arrow).
Figure┬Ā4.
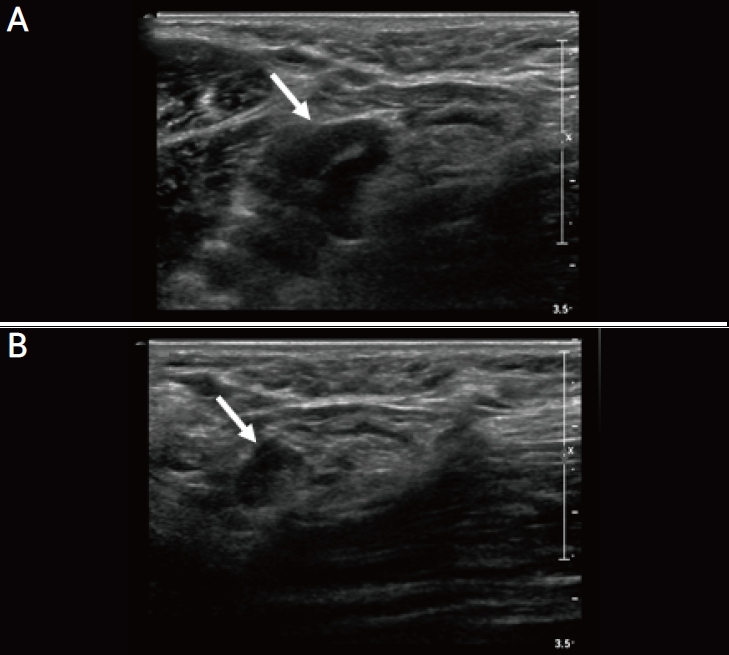
A 41-year-old woman with unilateral axillary lymphadenopathy after the first dose of Pfizer-BioNTech coronavirus disease 2019 vaccine. (A) Axial ultrasonography (US) images of the left axilla obtained within 4 days of vaccination show the cortex measuring up to 6 mm in the left axilla level 1 (arrow). (B) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 1.5 mm (arrow).

Figure┬Ā5.
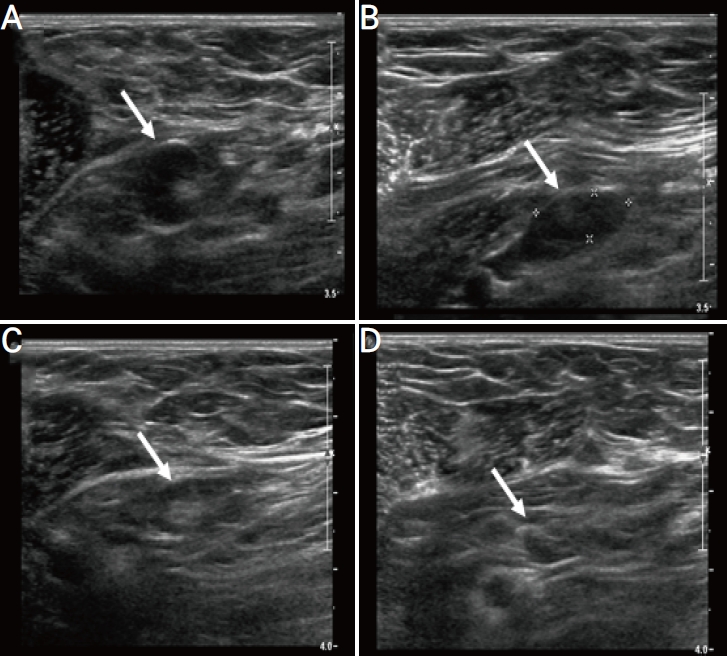
A 44-year-old woman with unilateral axillary lymphadenopathy after the first dose of Pfizer-BioNTech coronavirus disease 2019 vaccine. Axial ultrasonography (US) images of the left axilla obtained within 13 days of vaccination show the cortex measuring up to 6 mm and 5 mm in the left axilla (A) level 1 (arrow) and (B) level 2 (arrow), respectively. (C, D) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 2.5 mm (arrow).
Figure┬Ā6.
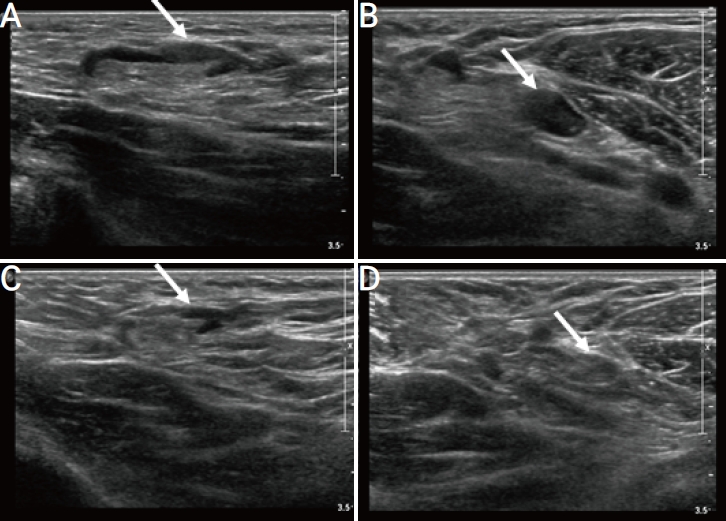
A 50-year-old woman with unilateral axillary lymphadenopathy after the first dose of Moderna coronavirus disease 2019 vaccine. Axial ultrasonography (US) images of the left axilla obtained within 14 days of vaccination show the cortex measuring up to (A) 10 mm (arrow) and (B) 5 mm (arrow). (C) Follow-up US images obtained within 6 months show overall decreased axillary lymphadenopathy but not fully resolved, with the cortex measuring up to 7 mm (arrow). (D) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 2 mm (arrow).

Figure┬Ā7.
A 30-year-old woman with unilateral axillary lymphadenopathy after the first dose of Oxford-AstraZeneca coronavirus disease 2019 vaccine. (A, B) Axial ultrasonography (US) images of the left axilla obtained within 2 days of vaccination show the cortex measuring up to 5 mm (arrow). (C, D) Follow-up US images obtained within 6 months show decreased axillary lymphadenopathy, with the cortex measuring up to 1.8 mm (arrow).
Table┬Ā1.
Clinical information and radiologic features of the eight patients
REFERENCES
1. Lehman CD, DŌĆÖAlessandro HA, Mendoza DP, Succi MD, Kambadakone A, Lamb LR. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol 2021;18:843-52.



2. National Center for Immunization and Respiratory Disease. Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events [Internet]. Atlanta: Centers for Disease Control and Prevention; c2021 [cited 2021 Jun 25]. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
3. National Center for Immunization and Respiratory Disease. The Moderna COVID-19 VaccineŌĆÖs Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events [Internet]. Atlanta: Centers for Disease Control and Prevention; c2021 [cited 2021 Feb 28]. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
4. Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphade-nopathy following COVID-19 vaccination: imaging findings review. Acad Radiol 2021;28:1058-71.



5. Dominguez JL, Eberhardt SC, Revels JW. Unilateral axillary lymphadenopathy following COVID-19 vaccination: a case report and imaging findings. Radiol Case Rep 2021;16:1660-4.



6. Ahn RW, Mootz AR, Brewington CC, Abbara S. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging 2021;3:e210008.



7. Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA vaccine with ipsilateral axillary lymph node reactivity on imaging. JAMA Oncol 2021;7:1241-2.



8. Faermann R, Nissan N, Halshtok-Neiman O, Shalmon A, Gotlieb M, Yagil Y, et al. COVID-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: analysis of 163 cases. Acad Radiol 2021;28:1191-7.



9. Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging 2021;75:12-5.



10. Wolfson S, Kim E, Plaunova A, Bukhman R, Sarmiento RD, Samreen N, et al. Axillary adenopathy after COVID-19 vaccine: no reason to delay screening mammogram. Radiology 2022;303:297-9.


11. Robinson KA, Maimone S, Gococo-Benore DA, Li Z, Advani PP, Chumsri S. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA Oncol 2021;7:1395-7.



12. Grimm L, Destounis S, Dogan B, Nicolson B, Dontchos B, Sonnenblick E, et al. SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination [Internet]. Okemos: Society of Breast Imaging; c2021 [cited 2021 Mar 14]. Available from: https://splf.fr/wp-content/uploads/2021/02/Society-of-breast-imaging-Recommendations-for-the-Management-of-Axillary-Adenopathy-in-Patients-with-Recent-COVID-19-Vaccination-Mis-en-ligne-par-la-Societe-francaise-de-radiologie-le-10-02-21.pdf.
13. Lane EG, Eisen CS, Drotman MB, Dodelzon K, Mema E, Thomas C, et al. Time for resolution of COVID-19 vaccine-related axillary lymphadenopathy and associated factors. AJR Am J Roentgenol 2022;219:559-68.


14. Lehman CD, Lamb LR, DŌĆÖAlessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol 2021;217:584-6.


15. Schiaffino S, Pinker K, Magni V, Cozzi A, Athanasiou A, Baltzer PAT, et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI). Insights Imaging 2021;12:119.



16. Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology 2021;300:E323-7.


17. Newfield L, Naschitz JE, Yeshurun D. BCG-induced axillary lymph-adenitis in the adult. Harefuah 1990;119:199-200.

18. Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines (Basel) 2021;9:147.



19. Lederer K, Casta├▒o D, G├│mez Atria D, Oguin TH 3rd, Wang S, Manzoni TB, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 2020;53:1281-95.e5.



20. Lane DL, Neelapu SS, Xu G, Weaver O. COVID-19 vaccine-related axillary and cervical lymphadenopathy in patients with current or prior breast cancer and other malignancies: cross-sectional imaging findings on MRI, CT, and PET-CT. Korean J Radiol 2021;22:1938-45.



21. Kim SJ, Park YM. Clinical importance and sonographic features of nonpalpable axillary lymphadenopathy identified on breast sonography in patients without malignancy. J Ultrasound Med 2015;34:2193-202.


22. Zhou W, DeMartini WB, Ikeda DM. Frequency and outcomes of ipsilateral axillary lymphadenopathy after COVID-19 vaccination. JAMA Netw Open 2022;5:e2216172.



23. Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 2021;106:376-81.



24. Yoshimoto N, Yanagi A, Takayama S, Sakamoto M, Tomoda K, Ishikawa K, et al. Timing and duration of axillary lymph node swelling after COVID-19 vaccination: Japanese case report and literature review. In Vivo 2022;36:1333-6.



25. Ha SM, Chu AJ, Lee J, Kim SY, Lee SH, Yoen H, et al. US evaluation of axillary lymphadenopathy following COVID-19 vaccination: a prospective longitudinal study. Radiology 2022;305:46-53.


26. Park JY, Lee JY, Yi SY. Axillary lymphadenopathy on ultrasound after COVID-19 vaccination and its influencing factors: a single-center study. J Clin Med 2022;11:238.



27. Co M, Wong PCP, Kwong A. COVID-19 vaccine associated axillary lymphadenopathy - A systematic review. Cancer Treat Res Commun 2022;31:100546.



28. Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, et al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol 2022;32:508-16.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,166 View
- 19 Download
- ORCID iDs
-
Jeong Jae Kim

https://orcid.org/0000-0003-0135-3804Su Yeon Ko

https://orcid.org/0000-0003-1405-3958 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print