|
 |
| J Med Life Sci > Volume 20(4); 2023 > Article |
|
Abstract
This study assessed risk factors for blood transfusion after Cesarean section in patients with partial placenta previa. We retrospectively reviewed the medical records of 149 patients who underwent Cesarean sections for partial placenta previa between January 2010 and October 2021. Clinical characteristics were compared between the two groups: the blood transfusion group (n=22), defined as patients who received a blood transfusion during surgery or within 24 hours after surgery, and the non-blood transfusion group (n=127), which included other patients. Multivariable logistic regression analysis identified two risk factors independently associated with blood transfusion: antenatal hemorrhage (adjusted odds ratio [aOR], 16.283; 95% confidence interval [CI], 4.405-60.190; P<0.001) and preoperative hemoglobin (g/dL) (aOR, 0.427; 95% CI, 0.246-0.739; P=0.002). Thus, patients who are at risk for these two factors should be carefully managed with sufficient preparation for blood transfusion and anesthetic management.
Placenta previa (PP) is an obstetric condition in which the placenta abnormally implants at the bottom of the uterus with complete or partial coverage of the cervix. PP can be associated with postpartum hemorrhage after Cesarean section (CS), which could induce maternal morbidity and mortality [1-3].
However, many clinicians consider postpartum hemorrhage relatively less common in patients with partial PP than in patients with complete PP [4,5]. Nevertheless, partial PP may trigger postpartum hemorrhage. Clinicians may lack preparation for the reservation of blood supplies, close patient monitoring, and other anesthetic management. Although several previous studies have reported risk factors for postpartum hemorrhage, such as degree of previa, anterior placental position, maternal age, and previous CS, most included both cases with complete PP and partial PP [6-11]. In contrast, few studies have been conducted on patients with partial PP. To address this knowledge gap, this study assessed risk factors for blood transfusion after CS in patients with partial PP.
We retrospectively reviewed the medical records of patients who underwent CS for partial PP at Jeju National University Hospital between January 2010 and October 2021. This study was approved by the institutional review board (IRB) of Jeju National University Hospital (IRB No. 2021-10-013). The IRB waived the requirement for obtaining informed consent.
In this study, twin pregnancies were excluded from the analysis. Because almost all CS procedures were performed by two specific obstetric surgeons, patients who underwent CS by other surgeons were excluded to reduce surgical bias.
The blood transfusion group (group B) was defined as patients who received packed red blood cells (RBCs) during CS or within 24 hours after CS. In contrast, those not receiving packed RBCs were assigned to the non-blood transfusion group (group N). Collected variables for univariable analysis included maternal age, height, weight, American Society of Anesthesiologists’ classification, gestational age, Apgar score, baby birth weight, previous abortion, multiparity, previous CS, previous uterine surgery except for CS, preoperative coagulation abnormality, uterine myoma, gestational diabetes, gestational hypertension or preeclampsia, antenatal hemorrhage, antenatal use of tocolytics, emergent CS, anterior placental position, and preoperative hemoglobin.
To compare variables between the two groups with and without blood transfusion, a chi-squared or Fisher’s exact test was performed for categorical data, and a Mann-Whitney test was performed for numerical data. Multivariable logistic regression analysis was then conducted by collecting variables with P<0.1 in univariable analysis. All data were analyzed using IBM SPSS Statistics version 20.0 (IBM, Armonk, NY, USA). Differences were considered statistically significant at P<0.05.
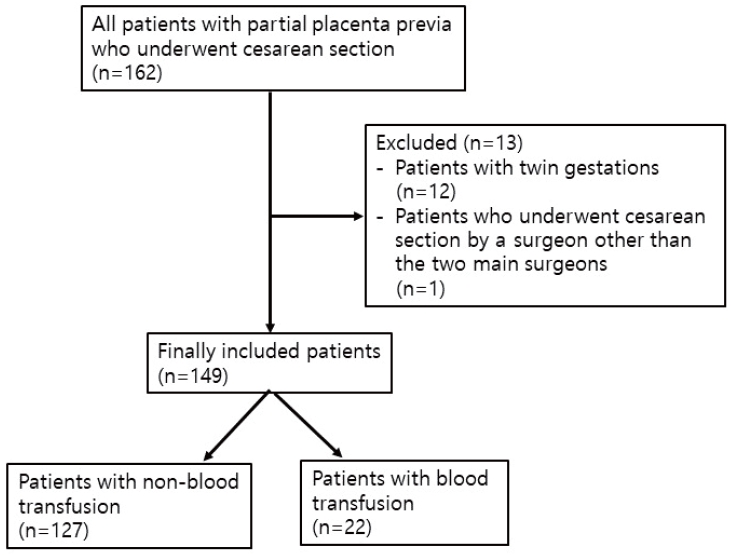
A total of 162 patients underwent CS for partial PP during the study period. Twelve patients with twin gestations were excluded from the analysis. One patient who underwent CS by a surgeon other than the two main surgeons was excluded (Fig. 1). As a result, a total of 149 patients were analyzed in this study. Of these, 22 (14.8%) required a blood transfusion. The median transfused packed RBCs in group B were two units. The median estimated blood loss was 600 mL in group N and 1,250 mL in group B, and the estimated blood loss of the patient with the most bleeding was 2,200 mL. This patient was transfused with eight units of packed RBCs.
No statistically significant difference was observed in the incidence of blood transfusions between the two surgeons. In group N, 123 patients (96.9%) received spinal/epidural anesthesia, and four patients (3.1%) received general anesthesia. In group B, 19 patients (86.4%) received spinal/epidural anesthesia, and three patients (13.6%) received general anesthesia or were converted to general anesthesia after spinal/epidural anesthesia (Table 1). Although patients in group B received general anesthesia more often than those in group N, we did not include the type of anesthesia in the multivariable analysis (see discussion for details).
In univariable analysis, variables including gestational age, previous abortion, multiparity, antenatal hemorrhage, antenatal use of tocolytics, emergent CS, and preoperative hemoglobin had P<0.1. They were thus included in the multivariable analysis (Table 2).
Multivariable logistic regression results show that risk factors independently associated with blood transfusion were antenatal hemorrhage (adjusted odds ratio [aOR], 16.283; 95% confidence interval [CI], 4.405-60.190; P<0.001) and preoperative hemoglobin (g/dL) (aOR, 0.427; 95% CI, 0.246-0.739; P=0.002) (Table 3).
PP, a significant obstetric complication, is associated with postpartum hemorrhage, for which the most commonly accepted risk factors are the degree of previa, anterior placental position, maternal age, and previous CS [7-10,12]. Among these, the degree of previa is considered the greatest risk factor. Many clinicians consider postpartum hemorrhage relatively less concerning in patients with partial PP. This conception leaves clinicians under-prepared for reserving blood supplies, close patient monitoring, and other anesthetic management.
In this study, 22 (14.8%) of the 149 patients with partial PP required blood transfusions. This incidence rate is similar to those in previous studies, in which 8-33% of patients with either complete or partial PP experienced blood transfusion after CS [8,13,14]. The patients who required blood transfusions needed prompt treatment, close monitoring, and the attention of high-level medical professionals. This highlights the need for efficient anesthetic management approaches, including identifying risk factors for blood transfusion in patients with even partial PP.
Oya et al. [13] previously reported maternal age, previous curettage, and degree of previa as risk factors for blood transfusion in patients with PP. Kang et al. [15] created a scoring model for predicting massive transfusion, including maternal age, degree of previa, anterior placental position, and sonographic hypoechoic layer in patients with PP. Lee et al. [16] reported that risk factors for massive postpartum hemorrhage in patients with partial PP included previous curettage, short cervical length, and uteroplacental hypervascularity. These conflicting results may be due to different compositions of the study population, such as whether or not patients with both complete and partial PP were included. Different definitions of postpartum hemorrhage or blood transfusion and evaluated factors could have affected the results.
This study focused on risk factors for blood transfusion in patients with partial PP. Our results demonstrate that antenatal hemorrhage and preoperatively lower hemoglobin levels constitute independent risk factors. While the placenta normally attaches at the top or on the side of the uterus, in PP, it attaches low in the uterus and covers the cervix. This can result in bleeding during pregnancy and, consequently, a need for surgery, even if the cervix is only partially covered [17,18]. Our study showed that 45.5% of the patients in group B experienced antenatal hemorrhage, whereas this was the case for only 3.9% of the patients in group N. Patients with antenatal hemorrhage may be at risk for lower preoperative hemoglobin levels, with a greater attendant chance of requiring a blood transfusion. Therefore, these patients should be carefully managed with sufficient preparation for blood transfusion.
In contrast, other risk factors, such as anterior placental position and previous CS, that were identified as important in previous reports [6,7,9], were not associated with blood transfusion in this study. Since these previous reports included patients with both complete and partial PP, it may be concluded that these factors have a greater influence on the former group of patients. However, the lack of studies comparing risk factors for blood transfusion in patients with complete or partial PP indicates that further research is needed to clarify this.
This study is subject to several limitations. Our retrospective review of medical records did not allow the identification of potentially incorrect records. Additionally, we considered blood transfusion based on the estimated blood loss, a hemoglobin level below 8-10 g/dL, and clinical findings such as cardiovascular changes. However, determining the timing of a blood transfusion might vary from doctor to doctor. Another limitation was that the incidence of general anesthesia or conversion to general anesthesia after spinal/ epidural anesthesia occurred more often in group B. In Jeju National University Hospital, CS is routinely performed under spinal/epidural anesthesia. In contrast, general anesthesia has been employed only in a few exceptional cases when the obstetric surgeon or anesthesiologist expects much bleeding or when the patient presents coagulation abnormalities. For this reason, the anesthetic method was not included as a factor in the analysis of this study. However, general anesthesia is considered a risk factor for postpartum hemorrhage [1,19,20].
In conclusion, our findings indicate that antenatal hemorrhage and preoperatively lower hemoglobin levels may constitute independent risk factors for blood transfusion after CS in patients with partial PP. Therefore, patients at risk from these factors should be carefully managed with sufficient preparation for blood transfusion and anesthetic management.
Acknowledgments
This work was supported by the 2023 education, research and student guidance grant funded by Jeju National University.
Table 1.
Perioperative characteristics in patients with and without blood transfusion
Table 2.
Clinical characteristics of patients with and without blood transfusions
REFERENCES
1. Butwick AJ, Ramachandran B, Hegde P, Riley ET, El-Sayed YY, Nelson LM. Risk factors for severe postpartum hemorrhage after Cesarean delivery: case-control studies. Anesth Analg 2017;125:523-32.



2. Lal AK, Hibbard JU. Placenta previa: an outcome-based cohort study in a contemporary obstetric population. Arch Gynecol Obstet 2015;292:299-305.


3. Crane JM, Van den Hof MC, Dodds L, Armson BA, Liston R. Maternal complications with placenta previa. Am J Perinatol 2000;17:101-5.


4. Tuzovic L. Complete versus incomplete placenta previa and obstetric outcome. Int J Gynaecol Obstet 2006;93:110-7.


5. Sekiguchi A, Nakai A, Kawabata I, Hayashi M, Takeshita T. Type and location of placenta previa affect preterm delivery risk related to antepartum hemorrhage. Int J Med Sci 2013;10:1683-8.



6. Hasegawa J, Matsuoka R, Ichizuka K, Mimura T, Sekizawa A, Farina A, et al. Predisposing factors for massive hemorrhage during Cesarean section in patients with placenta previa. Ultrasound Obstet Gynecol 2009;34:80-4.


7. Baba Y, Matsubara S, Ohkuchi A, Usui R, Kuwata T, Suzuki H, et al. Anterior placentation as a risk factor for massive hemorrhage during cesarean section in patients with placenta previa. J Obstet Gynaecol Res 2014;40:1243-8.


8. Jing L, Wei G, Mengfan S, Yanyan H. Effect of site of placentation on pregnancy outcomes in patients with placenta previa. PLoS One 2018;13:e0200252.



9. Na C, Kim HJ. Body mass index and massive hemorrhage after Cesarean section in patients with placenta previa. J Med Life Sci 2022;19:39-45.

10. Jang DG, We JS, Shin JU, Choi YJ, Ko HS, Park IY, et al. Maternal outcomes according to placental position in placental previa. Int J Med Sci 2011;8:439-44.



11. Park HS, Cho HS. Management of massive hemorrhage in pregnant women with placenta previa. Anesth Pain Med (Seoul) 2020;15:409-16.



12. Ogoyama M, Takahashi H, Baba Y, Yamamoto H, Horie K, Nagayama S, et al. Bleeding-related outcomes of low-risk total placenta previa are equivalent to those of partial/marginal placenta previa. Taiwan J Obstet Gynecol 2022;61:447-52.


13. Oya A, Nakai A, Miyake H, Kawabata I, Takeshita T. Risk factors for peripartum blood transfusion in women with placenta previa: a retrospective analysis. J Nippon Med Sch 2008;75:146-51.


14. Boyle RK, Waters BA, O’Rourke PK. Measures of blood loss and red cell transfusion targets for caesarean delivery complicated by placenta praevia. Aust N Z J Obstet Gynaecol 2010;50:242-5.


15. Kang J, Kim HS, Lee EB, Uh Y, Han KH, Park EY, et al. Prediction model for massive transfusion in placenta previa during Cesarean section. Yonsei Med J 2020;61:154-60.



16. Lee HJ, Lee YJ, Ahn EH, Kim HC, Jung SH, Chang SW, et al. Risk factors for massive postpartum bleeding in pregnancies in which incomplete placenta previa are located on the posterior uterine wall. Obstet Gynecol Sci 2017;60:520-6.



17. Hasegawa J, Nakamura M, Hamada S, Matsuoka R, Ichizuka K, Sekizawa A, et al. Prediction of hemorrhage in placenta previa. Taiwan J Obstet Gynecol 2012;51:3-6.


18. Bhide A, Thilaganathan B. Recent advances in the management of placenta previa. Curr Opin Obstet Gynecol 2004;16:447-51.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 698 View
- 22 Download
- ORCID iDs
-
Hyun Jung Kim

https://orcid.org/0000-0002-4852-7165Woo Hee Lim

https://orcid.org/0009-0004-1056-2266Young Sun Kim

https://orcid.org/0009-0004-7241-2183 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print